The Science Behind Micronutrients
The basic requirements for plant growth are generally the same for all higher plants, although the amounts vary widely. In addition to light, heat, water, and air, plants require a range of nutrient elements. Except for carbon, hydrogen, and oxygen, the soil is depended upon to supply these nutrients. The nutrients required for plant growth are divided into three broad categories, the major elements, the secondary elements, and the trace elements or micronutrients.

The Major Elements
The major elements are nitrogen (N), phosphorus (P), and potassium (K). Standard notation on commercial fertilizers will list these elements by the ratio of each in the product, such as 10-10-10 or 18-6-6. The Secondary Elements include calcium, magnesium, and sulfur. These are often added to the soil as "amendments" separate from fertilizers.
The Trace Elements
The trace elements or micronutrients are comprised of iron, manganese, copper, zinc, boron, and molybdenum. Analogous to vitamins, the micronutrients are utilized by plants only in small amounts. However, if even one of these elements are lacking or unavailable to the plant, growth will be severely inhibited, just as if it lacked all the other elements. Table 1 is a listing of the various elements and their functions in higher plants.
Element Availability in the Soil
A chemical analysis of any given soil will usually show the presence of the micronutrients. However, the particular physical and chemical characteristics of that same soil will determine whether the micronutrients are available to plants for absorption by their root systems. The pH of the soil is especially influential on element availability. For example, in soils with a pH above 7.0 (alkaline soils) iron availability to plants is significantly hampered by the soils' ability to "fix" (or "sequester") iron by chemically locking it into insoluble iron oxides. Conversely, molybdenum availability is limited when soils have a pH less than 7.0 (are "acidic").
Table 1
| Element | Function in Plant Growth | Function in Crop Production |
| Zinc | Activates growth hormone enzymes Enhances chlorophyll production Enhances respiration Transforms carbohydrates | Regulates growth Hasten plant maturity Aids carbohydrate formation Stimulates seed production Influences protein formation Promotes water absorption |
| Boron | Essential in cell wall formation Essential for translocation of sugars & starches Regulates starch production Aids in terminal bud formation Essential in formation of pollen grains & tubes | Essential for seed production Enhances protein production Aids in nodule formation Enhances disease resistance |
| Manganese | Regulates growth hormone supply Acts as a catalyst Activates enzymes Aids in photosynthesis Aids in respiration | Accelerates germination Hastens maturity Regulates uptake of C, Mg, & P |
| Copper | Aids in chlorophyll synthesis Acts as a catalyst Activates enzymes Aids in photosynthesis Aids in respiration | Enhances nitrogen utilization Stimulates protein formation Functions in root metabolism |
| Iron | Essential for respiration Aids in chlorophyll synthesis | Essential for healthy growth |
| Molybdenum | Enzyme catalyst for reducing nitrates to ammonia Converts inorganic P to organic form | Enhances nodule formation Enhances nitrogen fixation Enhances protein formation |
After "Trace Elements in Soils & Crops", N.H. Pizer, ed.
Figure 1
Figure 1 shows the range of important elements and their relative sequestration under varying soil conditions. In this chart, the larger colored areas indicate that the soil will "fix" that element, thus making it unavailable to the plant.
Method of Supplying the Trace Elements
Most often, the method of supplying the trace elements to the soil has been to add the soluble salts of these elements as a direct soil amendment or by inclusion in a standard NPK fertilizer blend. That is, the metal ion in a SULFATE compound (SO4) is added. However, there are two significant problems with this approach. First, adding the sulfate complex of the metal does nothing to address the ability of the soil to sequester the element. For example, adding iron sulfate to alkaline soils will allow the following chemical reaction:
Fe2SO4 + O2 + H2O → Fe2O3 + H2SO4
That is, iron sulfate plus oxygen plus water produces iron oxide (rust) plus sulfuric acid. Most of the other metal sulfates have a similar reaction.
Secondly, when the trace metals are tied into sulfate compounds, most of the elements are not available to the plant. The ability of plants to directly absorb and utilize the sulfate forms of the trace metals is limited at best. The grower's response to this in the past has been to simply add more sulfate, leading to accumulations in the soil that eventually may reach toxic levels.
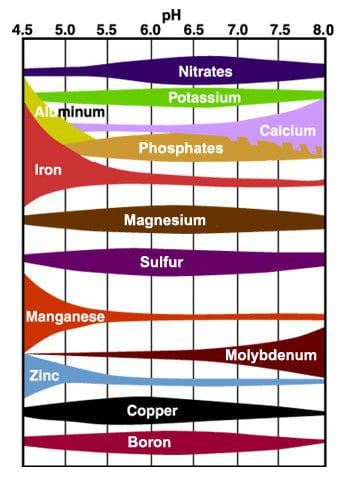
After "Soils and Soil Fertility," Troeh & Thompson, 1993.
Listed below in Table 2 are some of the general symptoms of micronutrient deficiency in plants.
| Element | Symptoms of Deficiency | Common Soil Types |
| Iron | Yellowing between the veins or ribs of younger leaves. Eventual loss of color and turgidity to the entire plant. | Iron is sequestered in neutral to alkaline soils, and in acidic soils where excessive amounts of soluble phosphates are present. Precipitation of iron phosphate is more likely to occur in sandy rather than clay soils. |
| Manganese | Loss of color to the leaf between veins. The resulting color may be yellow to almost white. Small gray specks appear on young leaves first, then eventually the entire plant. | Manganese deficiency is associated with neutral to alkaline soils. |
| Copper | Young leaves are permanently wilted, and the growth of the entire plant is retarded. Often, there is die-back in citrus, with bumpy and split fruit. | Copper deficiency is often associated with soils high in organic matter. |
| Zinc | Leaves are mottled to uniformly yellow in color. | Zinc deficiency is most often associated with sandy, alkaline soils. |
| Molybdenum | A distinctive mottling occurs in older leaves, with veins remaining light in color. As the deficiency is prolonged, puffing of the chlorotic areas occurs. Leaves curl inward and begin to die along tips and margins. | Molybdenum deficiency is often associated with soils high in organic matter. |
| Boron | Plant stems become brittle. Leaves will often curl inward and produce a burnt appearance. Growth is stunted or gnarled. | Boron deficiency can occur in either strongly acidic or alkaline soils. |
After "Hunger Signs In Crops", Howard B. Sprague, ed.
Chelated Micronutrients
To avoid the problems of sequestering, toxic accumulation, and non-absorption, we turn now to CHELATED micronutrients. Plant and soil scientists have shown that the chelated form of a trace element can be up to 450 times as effective as the sulfate form when used as a soil amendment (Georgia-Pacific Corp).
The term CHELATED means that a trace metal ion (positive molecular charge, or cation) is combined with electron donor groups (negative molecular charge, or anion) from another molecule. That molecule is called the CHELATE. Many substances will chelate metal ions. However, the choice of chelate for use in agriculture and horticulture depends upon several factors. Firstly, the element must be absorbable by the plant. Second, the chelate must be non-toxic to the plant. Third, the chelate should be a molecule that the plant can either remove from its system or utilize in its structure. And fourth, the chelate needs to be stable in the soil.
Chelating Agents
There are several chelating agents available on the market that, to some degree or another, would fulfill the requirements listed above. The most popular ones include Citric Acid, Glucoheptonate, Lignosulfonate, and EDTA. Their relative chelating power and availability for plant uptake, when compared to sulfates, are as follows:
At first blush, it would appear to be most effective to use EDTA and DPTA as a chelating agent. However, they are specifically excluded from being certified as an organic material under the National Organic Program (USDA NOP Rule 205.105). In addition, EDTA, DPTA, and citric acid are materials that are not normally part of a plant's structure (non-citrus) and thus must be eliminated through plant metabolism to not accumulate in the plant. Further, they are relatively high-cost materials when compared to lignosulfonate.
Lignosulfonates Revealed
Very generally, trees and all higher plants are comprised of two basic substances – CELLULOSE, which makes up the "building block" of the plant, and LIGNIN, which is the "cement" that holds the blocks together. Figure 2 is a stylized diagram of the complex lignin molecule.
When wood is harvested for the paper and pulp-making process, it is in the form of 2-3" diameter flat chips. These chips are cooked in large boilers under acidic conditions, separating the cellulose from the lignin. The cellulose is removed to be further processed into paper products.
In the past, the remaining lignin solution (now converted to lignosulfonate) was either dumped into a waste pond or burned as fuel for the boilers.
But in the early 1960s, a pioneering group of scientists as Courtesy of the Lignin Institute, Atlanta, GA
The Georgia-Pacific Corporation in Bellingham, Washington began to develop ways to take advantage of the wide-ranging characteristics of this natural substance.
Despite the apparent complexity of the lignosulfonate molecule, the chemical characteristics are relatively easy to understand. Lignosulfonates work like this:
Negatively charged lignosulfonate molecules readily absorb positively charged particles or surfaces with less charge density. The lignosulfonate can thus be modified to neutralize or enhance the surface charge of the resulting compound, making them excellent materials for use as flocculants, dispersants, binders, or chelates.
Some of the amazing variety of industries and products in which lignosulfonates are utilized include:
Thus, what was once a waste stream suitable only for dumping or burning is now an integral part of the structure of our world.
Figure 2